Wp/dtp/Atom
| Atom | ||||||
|---|---|---|---|---|---|---|
| 300px|right|Kowoowoyoon do atom helium | ||||||
| Kopokitanan sampapas kosudong kokomoi struktur atom helium. Kotuongon tawan elektron nopo nga' misudong do kamiran garis okito id fungsi kebarangkalian orbital elektron di kumoiso. Nukleus atom ii pinoingayo diti nopo nga' kikowoowoyoon do skematik, miampai proton di kiwarana buragang om neutron kiwarana osohug. Miampai nyata, nukleus (om fungsi gelombang monikid nukleon) nogi kibontuk do sfera miampai kisimetri. (Haro pisuayan montok kes-kes nukleus kompleks.) | ||||||
| Pomoogian kawo |
| |||||
| Kowoowoyoon | ||||||
|
Id gana do kimia om fizik, atom (Boros Yunani) άτομος toi ko' átomos nopo nga' kirati do "amu milo boogion") nopo nga' zarah bobos tokoro ii milo onuon id suang do roromu kimia miampai amu monimban do kowoowoyoon kimia dau. Hogot atom nopo nga' kirati do zarah amu milo boogion di bobos tokoro ii milo onuon, nga' kalapas istilah dii nakaanu rati khusus id sains, atom-atom di norubaan milo boogion po kawagu om otutunan sabaagi zarah subatom.
Kogumuan atom nopo nga' haro tolu kawo zarah subatom ii papatantu do kowoowoyoon id soliwan:
- elektron, kiharo cas negatif om nogi bobos tokoro mantad kotolu-tolu zarah diti
- proton, kiharo cas positif om lobi kurang 1836 kali lobi agayo mantad do elektron; om
- neutron, aiso cas om lobi kurang 1839 kali lobi agayo mantad ko' elektron.
Proton om neutron nopo nga' miamung mombontuk do nukelus atom ii opidot om agayo, om piomungan diti nopo nga' lohowon sabaagi do nukleon. Elektron-elektron diti mombontuk tawan elektron di lobi agayo id posorili do nukleus.
Monikid atom nopo diti nga' mogisuusuai ginumu do zarah-zarah subatom. Ginumu proton id suang do atom ((lohowon sabaagi numbur atom) papatantu do roromu atom dii. Mantad do roromu-roromu di miagal dii, ginumu neutron nogi nga' milo misiisimban, om iri papatantu do isotop roromu dii. Ginumu proton om neutron id suang do nukleus atom nogi nga' milo sumimban, maya pamalapakan nukleus, pelakuran nukelus om koromukon radioaktif. Ginumu elektron di kipionitan do isoiso atom nopo nga' bobos osonong do sumimban tu' tenaga di osiriba di gunoon montok mongogos do monikid elektron.
Atom nopo nga' neutral maya elektik sokiro haro ginumu proton om elektron di miagal. Atomm ii nakawaya pongingkurian toi ko' pomoruhangan do elektron nopo nga' lohowon sabaagi do ion. Elektron di bobos tosodu mantad do nukleus milo poolihon kumaa atom di miinsomok toi ko' miilang do atom suai. Maya mekanisme atom diti nopo nga' haro pikagasan ii sumiliu do molekul om kawo suai do sebatian kimia miagal do hablur kirangkaian ionik toi ko' kovalen. Montok gas om piipiro cecair om pepejal molekul (miagal do waig om gula), molekul nopo nga' pomoogian do jirim bobos tokoro ii popotindohoi do kowoowoyoon kimia; nga', haro nogi ogumu po pepejal om cecair mantad do atom-atom, om aiso suang do molekul mitongkiad (poomitanan nopo nga' tusi, watu om logam cecair om pepejal). Kogumuan molekul nopo nga' owonsoi mantad mogikaakawo atom; poomitanan nopo nga' molekul waig di owonsoi mantad piomungan duo atom hidrogen om iso atom oksigen. Piipiro kawo molekul (miagal di roromu molekul gas di amu kobontuk do sebatian, miagal do helium), owonsoi mantad iso kawo do atom.
Atom nopo nga' blok impohon pamansayan id suang do kimia, om nogi terabadi id suang do tindak balas kimia.
Sajara Koinlaaban Model Atom[edit | edit source]
Hogot atom nopo nga' naanu mantad boros Yunani ii kirati do iso ii amu no milo do babakon.Democritus (460 - 357 S.M) nopo nga' tulun kumoiso di pinopointutun do konsep atom. Iti nopo nga' kopiagal do teori Dalton di pinopoilo do atom nopo nga' zarah bobos tokoro om amu no milo piboogion.
Ilo nopo nga' puru kimia ii minanahak kouhupan id suang koinlaaban model atom:
Keistimewaan Atom Karbon[edit | edit source]
Atom karbon miampai numbur atom 6 nopo nga' haro nuludan elektron K = 2, L = 4, om haro 4 elektron valens om kaanu mombontuk apat kogos kovalen, om nogi milo pokitonon id suang rumus Lewis sabaagi dilo, miagal do montok CH4.
kogos id suang molekul metana [gambar] atom karbon
[gambar] 4 atom hidrogen
[gambar] molekul metana ( CH4 ) [gambar] diagram sederhana mantad molekul metana
thumb|Molekul metana dalam 2D.
H H \ / C / \ H H
apat kogos kovalen mantad molekul metana Suai ko' iri, karbon nopo nga' milo mombontuk kogos miampai atom karbon suai do mombontok rantai akrbon di poingukab toi ko' poinsompon, poinrilit. Poomitanan nopo rantai karbon nga' milo intangan id suang do rumus struktur:
| | | | |
- C - C - - C - C - C -
| | | | | C
rantai poingukab rantai poingukab om mirapang
| |
- C - C -
| |
- C - C -
| |
rantai poinsompon Dondo nosimbar no nokuro ginumu senyawa karbon irid idi ginumu hali pia ginumu kawo roromu pomobontuk nopo nga' okuri.
Atom id suang do falsafah[edit | edit source]
Idea nopo kokomoi kakamot nga' mantad do unit pointantu nopo nga' idea di nakalaid no, ii haro id kogumuan koubasanan dongulu-gulu miagal do Greek om India. hogot "atom" nopo nga' winonsoi do puru falsafah Yunani purba. Hali pia miagal di, tua pomusarahan diti nopo nga' poingimpohon do pomusarahan falsafah om teknologi om okon ko tumanud do bukti om ponoriukan. Asil mantad dii, tua' pomusarahan diolo kokomoi poingkuro upa atom kowoowoyoon NOPO NGA' amu otopot. Amu nogi yolo kaanu popotumboyo toinsanan tulun, mantad dii, tutumanud atom nopo nga' iso mantad piipiro teori kokomoi kowoowoyoon jirim di misaing. Antakan di abad ko-19, notorimo om pinoinsonong nogi idea di maya do tongosaintis, soira koinlaaban sains kimia nakapaasal kinabantalan di kaanu no potolinahason maya konsep atom.
Teori koiso tumanud do bukti[edit | edit source]
Ontok di timpuunon toun 1800-an, John Dalton minomoguno konsep atom montok popotolinahas nokuro unsur nopo nga' isaru manahak tisuli id suang do nisbah numbur tokoro (hukum mogikaakawo perkadaran). Sabaagi poomitanan, haro duo kawo oksida timah: iso nopo nga' 88.1% timah om 11.9% oksigen om ii suai nopo nga' 78.7% timah om 21.3% oksigen (timah (II) oksida om tin dioksida monikid tiso). Iti nopo nga' kirati do 100g tin nopo nga' maimung miampai 13.5g toi ko' 27g oksigen. 13.5 om 27 nopo nga' mombontuk do nisbah 1:2, iso nisbahh numbur bulat tokoro. Iti nopo nga' wotik koubasanan id suang do kimia ii pinoposogu di Dalton do roromu-roromu manahak tisuli id suang gandaan numbur bulat unit diskret-toi ko' id boros suai nopo nga' atom. Id suang do kes oksida timah, iso atom timah nopo nga' miamung miampai iso toi ko' duo atom oksigen. [1]
Otumbayaan nogi i Dalton do teori atom nopo nga' milo popotolinahas nokuro waig nopo nga' moniop do gas-gas di mogisuusuai id suang do perkadaran di misuai. Sabaagi poomitanan, nokoilo idiso do waig nopo nga' monosop karbon dioksida lobi osonong mantad ko' monosop do nitrogen.[2] Minanahak i Dalton do andaian hipotesis diti sabap do haro pisuayan do jisim om tatarajah zarah monikid gas, om molekul karbon dioksida (CO2) nopo nga' lobi aagat om lobi agayo suai ko' molekul nitrogen (N2).
Koguraan Brownian[edit | edit source]
Ontok di toun 1827, songulun puru botani, Robert Brown nopo nga' minomoguno mikrosop montok mogintong butiran debu ii poinghampung id suang do waig om nakaanu do iri nopo nga' minggura miampai olias, iso fenomena ii otutunan sabaagi do "gerakan Brownian". Iti nopo nga' sabap do molekul waig nopo nga' manahub do butiran merata. Om ontok di toun 1905, Albert Einstein pinopobukti realiti molekul om koguraan diolo miampai pinapaasil do analisis kumoiso fizik statistik montok koguraan Brownian. [3][4][5] Songulun puru fizik Perancis, Jean Perrin minomoguno do karaja Einstein montok momonsoi poniisan montok papatantu jisim om dimensi atom, om miampai muktamad pinoposah do teori atom Dalton.[6]
Kinorubaan do elektron[edit | edit source]
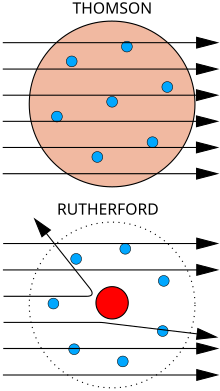
Atas: Hasil dijangka: Partikel alpha melalui menerusi model puding plum bagi atom dengan lencongan minima.
Bawah: Hasil dilihat: sebilangan kecil partikel dilencongkan oleh tumpuan caj positif bagi nukleus.
Songulun puru fizik J. J. Thomson minonipong do jisim pancaran katod, pinopobukti do iri nopo nga' obontuk mantad do partikel, nga' 1800 kali ganda lobi agaan mantad ko' atom bobos tagaan, hidrogen. Miampai dii, iri nopo nga' okon ko' atom, nga' partikel wagu, partikel sub-atom koiso di norubaan, ii roitan sabaagi do "corpuscle" nga' pinungaranan kawagu do elektron, sempena partikel di naaga di George Johnstone Stoney ontok di toun 1874. Pinopobukti nogi isio do iri nopo nga' miagal miampai partikel di nakaasil do kakamot fotelektrik om radioaktif.[7] Iri nopo nga' oruhai do nopurimanan do partikel ii mogoit do arus lotirik id suang do wayar logam, om mogoit do cas negatif lotirik id suang do atom. Noonuan do Tutungkap Nobel i Thomson montok Fizik 1906 montok ponoriukan dau. Mantad dii, pinopoguli isio do kotumbayaan kokomoi atom nopo nga' jisim partikel ulung, ii amu milo boogion.[8] Thomson nogi nga' nokosilap do minangaga do elektron kicas negatif kijisim do tosiriba nopo nga' tersebar id suang do atom ii kisuang do cas positif di miagal. Iti nopo nga' nointutunan sabaagi model puding plum.
Kinorubaan do nukleus[edit | edit source]
Ontok di toun 1909, Hans Geiger om Ernest Marsden, id siriba do ponyoliaan di Ernest Rutherford, monghujani pinayanan logam do zarah alfa montok mogintong poingkuro iri mogiuriasan. Minangaga yolo do toinsanan zarah alpa nopo nga' katalib do apanggor miampai okuri kosimbanan, tu' model Thomson nakarait do caj id suang do atom nopo nga' mogiuriasan gisom medan lotirik diolo amu kaanu do manangkangau zarah alfa di ogumu. Hali pia miagal dii, Geiger om Marsden nakaanu do zarah alfa ii pinopesong do sudut di lobi agayo, kolobi do 90°, ii sepatutnya kosiliu musatahil tumanud do model Thomson. Montok popotolinahas diti, pinoposogu i Rutherford do caj positif atom pointumpu id nukleus di tokoro id pintangaan do atom.[9] Rutherford pinopiadang do kinorubaan disio miampai iso tembakan pinulu 15 inci om rinumikot kawagu montok mongontok tulun di minonimbak dii.[10]
Kinorubaan do isotop[edit | edit source]
Maamaso do minongumbal do asil kinoromukan radioaktif, ontok di toun 1913, puru radioaktif, i Fredrick Soddy nokoruba do iri nopo nga' nokitanan lobi ko' iso kawo atom id monikid koilihan id jadual berkala.[11] Istilah isotop nopo nga' winonsoi di Margaret Todd sabaagi ngaran di kosudong montok atom di misuai, i poinsuang id roromu di miagal. J. J. Thompson nogi nga' minomonsoi teknik montok popisuai do kawo atom maya ponoriukan disio kokomoi gas terion, ii minogoit do kinorubaan Isotop stabil.[12]
Model Bohr[edit | edit source]

Ontok di toun 1913, songulun ahli fizik, Niels Bohr pinoposogu do iso model hinonggo elektron atom naanggap do mintutuk do nukleus nga' milo no momonsoi miagal dii id iso set orbit terhad, om milo sumimpok id pialatan do orbit diti maya kosimbanan diskret tenaga di miagal maya panasapan toi ko' sinaran foton.[13] Pengkuantuman diti nopo nga' noguno montok popotolinahas nokuro orbit elektron stabil (sabap koubasan nopo nga', caj memecuk, kohompit no do koguraan bumulugu, katagakan tenaga kinetik ii pinancar sabaagi radiasi elektromagnet, intangai radiasi sinkrotron) om nokuro roromu monosop om papapancar do sinaran elektromagnet id suang do spektrum diskret.[14]
Kalapas dii, ontok di toun di miagal, minanahak i Henry Moseley do eksperimen pomoruhang ii memihak kumaa teori Niels Bohr. Koutuson diti pinoinsonong do model Ernest Rutherford om Antonius Van Den Broek, ii pinoposogu do atom ii poinsuang id nukleus soginumu caj nuklear positif di miagal do numbur (atom) id suang do jadual berkala. Gisom do ujikaji diti, numbur atom amu noilaan sabaagi do ginumu fizikal om eksperimental. Iti nopo nga' kopiagal do caj nuklear atomik sabaagi do model atom di otorimo gisom dinondo.[15]
Kotolinahasan do pangagasan kimia[edit | edit source]
Kogos kimia id pialatan do atom diti nopo nga' nokotolinahas di Cilbert Newton lewis ontok di toun 1916, sabaagi interaksi do elektron konstituen diolo.[16] Tu' kowoowoyoon kimia montok roromu nopo nga' oilaan do soboogian ginayo nopo nga' mongohulit diolo sondii tumanud do undang-undang berkala,[17] ontok di toun 1919, songulun ahli kimia Amerika, Irving Langmuir pinoposogu do iri nopo nga' milo potolinahason sokiro elektron id suang do atom nopo nga' nokopioput toi ko' tinimungan bontuk pointantu. Tinimungan elektron notumbayaan do haro id iso set kelongsong elektron sumorili do nukleus dii.[18]
Koinlaaban id suang do fizik kuantum[edit | edit source]
Poniisan Stern-Gerlach ontok di toun 1922 nopo nga' minanahak do iso bukti kokomoi kowoowoyoon kuantum atom. Soira pancaran atom perak nakawaya do medan magnet kibontuk khas, pancaran dii ababak tumanud do koguraan do sudut zarah, toi ko' turugan. Sabap do koongoyon nopo nga' rawak,pancaran nopo nga' aaga kopulih id iso barisan. Suai ko iri, pancaran diti nogi nga' ababak kumaa duo boogian, tumanud sama ada turugan do atom noorientasi id sawat toi ko' id siriba.[19]
Ontok di toun 1924, Louis de Broglie pinoposogu do toinsanan zarah okuri ogumu kikowoowoyoon miagal do lakun. Ontok di toun 1926, Erwin Schrödinger, minomoguno idea diti montok popoinlaab model Matematik montok atom ii kikowoowoyoon elektron sabaagi bontuk lakun tolu dimensi, om okon ko' zarah kititik. Asil momoguno iso bontuk lakun montok popokito zarah nopo nga' iri nopo nga' mustahil miampai matematik montok maganu gatang di koontok montok koduo-duo kedudukan om momentum zarah id titik pontantu id timpu pointantu; otutunan sabaagi prinsip aiso kabayaan, ii pinolingkum di Werner Heisenberg ontok di toun 1926. Id suang do konsep diti, montok kotopoton ii pinatahak montok monipong kedudukan soosongulun nopo nga' milo no onuon mantad julat gatang kikoduyaan montok momentum, om sebaliknya.[20] Model diti popotolinahas do pongintangan kowoowoyoon atom di amu milo powonsoyon di model laid, miagal do struktu om pola spektrum montok atom di lobi agayo kopiadang do hidrogen. Mantad dii, model planet montok atom noidu om nalanan do nunu ii kaanu popokkito zon orbital atom sumorili do nukleus hinonggo elektron pointantu kikoduyaan tagayo milo intangan.[21][22]
Kinorubaan do neutron[edit | edit source]
Kinoingkakaton do spektrometri jisim pinapasaga jisim atom milo tipongon do otopot. Peranti diti minomoguno do magnet montok popolencong do trajektori pancaran ion, om ginumu pesongan pinatantu do nisbah jisim atom poingadang do cas dau. Songulun ahli kimia, Francis William Aston minomoguno do kakamot diti montok popokito do Isotop nopo nga' haro jisim di misuai. Jisim atom di mogisuusuai montok isotop diti nopo nga' tumanud do ginumu integer, lohowon sabaagi kooturan numbur bulat. [23] Kotolinahasan montok isotop di mogisuusuai diti minagandad do kinorubaan do neutron, zarah kicas om jisim di miagal, miagal do proton, i naanu di ahli fizik, James Chadwick ontok di toun 1932. Isotop nopo antakan dii nga' potolinahason sabaagi do roromu-roromu miampai numbur proton di miagal, nga' numbur neutron di misuai id suang do nukleus.[24]
Pamalapakan, fizik kitenaga takawas om winagat di opidot[edit | edit source]
Ontok di toun 1938, songulun ahli kimia Jerman, Otto Hahn, sngulun mokiikinobos di Rutherford, mogintong neutron id atom uranium om mangaga montok maganu roromu transuranium. Sebaliknya, eksperimen kimia disio pinopokito do barium sabaagi do asil.[25][26] Sontoun kalapas dii, Lise Meitner om kamanakon di Otto Frisch pinoposah do asil di Hahn nopo nga' eksperimen pamalapakan nuklear koiso.[27][28] Ontok di toun 1944, nokotorimo i Hahn do Nobel id gana do kimia. Id tolikud do goos di Hahn diti, sumbangan di Meitner om Frisch nopo nga' amu noiktiraf.[29]
Ontok di toun 1950-an, kinoingkakaton do pemecut zarah versi di lobi osonong om mogigintong zarah pinasapasaga do ahli saint montok momoriuk bagas atom momogura momoguno tenaga di lobi akawas.[30] Neutron om proton nopo nga' aanu sabaagi do hadron, toi ko' komposit zarah di lobi okoro ii lohowon sabaagi do kuark. Model stndard fizik zarah di nokoingkakat setakat dinondo nopo nga' nakalantoi do popotolinahas kowoowoyoon nukleus mantad pogintangan zarah sub-atom om kuasa-kuasa ii mangawal interaksi diolo.[31]
Intangai nogi[edit | edit source]
Sukuon[edit | edit source]
- ↑ Andrew G. van Melsen (1952). From Atomos to Atom. Mineola, N.Y.: Dover Publications. ISBN 0-486-49584-1.
- ↑ Dalton, John. "On the Absorption of Gases by Water and Other Liquids", in Memoirs of the Literary and Philosophical Society of Manchester. 1803. Retrieved on August 29, 2007.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedadp322_8_549 - ↑ Mazo, Robert M. (2002). Brownian Motion: Fluctuations, Dynamics, and Applications. Oxford University Press. pp. 1–7. ISBN 0-19-851567-7. OCLC 48753074.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedlee_hoon1995 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namede31_2_50 - ↑ Thomson, J. J. (August 1901). "On bodies smaller than atoms". The Popular Science Monthly. Bonnier Corp.: 323–335. Linoyog ontok 2009-06-21.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namednobel1096 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpm21_669 - ↑ "The Gold Foil Experiment". myweb.usf.edu. Archived from the original on 2016-11-19. Linoyog ontok 2017-07-02.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namednpc1921 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedprsA_89_1_1913 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedstern20050516 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedbohr19221211 - ↑ Pais, Abraham (1986). Inward Bound: Of Matter and Forces in the Physical World. New York: Oxford University Press. pp. 228–230. ISBN 0-19-851971-0.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedjacs38_4_762 - ↑ Scerri, Eric R. (2007). The periodic table: its story and its significance. Oxford University Press US. pp. 205–226. ISBN 0-19-530573-6.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedjacs41_6_868 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedfop17_6_575 - ↑ TED-Ed (16 September 2014). "What is the Heisenberg Uncertainty Principle? - Chad Orzel" – via YouTube.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedbrown2007 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedharrison2000 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpm39_6_449 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedchadwick1935 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedBowden - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedCHF - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namednature143_3615_239 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedschroeder - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpt50_9_26 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedkullander2001 - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namednpp1990
Template:Wp/dtp/Tunas-fizik Template:Wp/dtp/Tunas-kimia Kategori:Sains Kategori:Sains tulen Kategori:Fizik Kategori:Kimia Kategori:Konsep fizik asas
